The core engine in the field of medical electronics: high reliability PCBA technology and applications As the cornerstone of modern electronics industry, PCBA (Printed Circuit Board Components) plays a key role in driving technological innovation. Especially in the healthcare industry, its value is highlighted as the industry undergoes transformation - China's healthcare system is experiencing a leapfrog development from traditional Chinese medicine to modern Western medicine, directly driving a compound annual growth rate of 18% in demand for medical electronic devices (data source: 2024 China Medical Device Blue Book).
Special breakthrough in medical grade PCBA
Different from ordinary consumer electronics, medical PCBs need to meet the following requirements:
Extreme environmental stability: -40 ℃~125 ℃ wide temperature range working ability
Life critical reliability: Mean Time Between Failures (MTBF) exceeding 50000 hours
Precision signal processing: supports μ V level bioelectric signal acquisition
C-alley's Medical Intelligent Manufacturing Practice
C-alley, which has passed the ISO 13485 medical quality management system certification, has demonstrated its technological advantages in:
Provide a 0.1mm pitch BGA packaging solution for ultrasound diagnostic equipment
Realize 99.998% signal transmission integrity in the monitor PCBA
Customize medical imaging equipment circuit modules through 3D printing technology
The healthcare and medical PCB applications we serve:
C-alley manufactures and assembles medical PCBs for various medical devices, such as:
medical image equipment
Medical monitoring system
robotic surgery
Radiotherapy or radiation therapy
Real time vital sign monitoring system (ECG/blood oxygen/respiration)
Digital imaging equipment (DAS data acquisition module for CT/MRI)
Intelligent surgical robot motion control unit
How to Choose the Suitable PCB for Medical Applications
Selecting the right Printed Circuit Board (PCB) for medical devices is critical due to the industry's stringent requirements for reliability, safety, and precision. Below are key considerations to guide your choice:
1. Compliance with Medical Standards
Ensure the PCB meets ISO 13485 (Quality Management for Medical Devices) and IEC 60601 (Safety Standards for Medical Electrical Equipment).
Certifications like UL 94 (flammability rating) and RoHS/REACH (restriction of hazardous substances) may also apply.
2. Material Selection
High-Frequency Materials (e.g., Rogers, Teflon) are ideal for imaging equipment (MRI, ultrasound) due to low signal loss.
FR4 is cost-effective for general medical electronics but may lack performance in high-reliability applications.
Flex/Rigid-Flex PCBs are suited for wearable or implantable devices, offering durability in compact designs.
3. Design for Reliability
High-Density Interconnect (HDI) technology is preferred for complex, miniaturized devices (e.g., pacemakers).
Redundancy and Fault Tolerance: Critical devices (e.g., ventilators) may require duplicate circuits to prevent failure.
Thermal Management: Use materials with high thermal conductivity (e.g., metal-core PCBs) for power-intensive equipment.
4. Manufacturing and Testing
Cleanroom Production: Essential for implantable devices to avoid contamination.
Advanced Testing: Include Automated Optical Inspection (AOI) and Electrical Testing to ensure zero defects.
Longevity: Medical PCBs often need a lifespan of 10+ years, requiring robust solder masks and coatings.
5. Supplier Expertise
Partner with manufacturers experienced in medical-grade PCBs, offering traceability and documentation (e.g., IPC Class 3 standards).

Main Types of PCBs for Medical Devices
Medical devices require highly reliable PCBs tailored to specific applications. The key types include:
1.Rigid PCBs
Most common in stationary equipment (e.g., MRI machines, monitors).
Made from durable materials like FR4 or high-frequency laminates (e.g., Rogers) for signal integrity.
2.Flexible PCBs
Used in wearable devices (e.g., ECG patches) and endoscopes.
Lightweight, bendable, and resistant to vibration.
3.Rigid-Flex PCBs
Combine rigid and flexible layers for compact, high-performance devices (e.g., pacemakers, surgical robots).
Reduce wiring errors and improve space efficiency.
4.High-Density Interconnect (HDI) PCBs
Essential for miniaturized implants (e.g., neurostimulators).
Feature microvias and fine traces for high-speed signal transmission.
5.Metal-Core PCBs
Used in high-power devices (e.g., X-ray machines) for superior heat dissipation.
Aluminum or copper substrates prevent overheating.
Key Considerations for Medical PCB Assembly
Medical PCB assembly demands extreme precision and reliability due to its critical role in patient care. Here are the top considerations:
1. Regulatory Compliance & Standards
Must meet ISO 13485 (quality management) and IEC 60601 (safety for medical devices).
FDA/CE approval may be required for certain markets.
RoHS/REACH compliance ensures hazardous material restrictions.
2. Material & Design Integrity
High-reliability substrates (e.g., FR4, Rogers, polyimide) for durability.
HDI (High-Density Interconnect) for compact, high-performance devices.
Thermal management (e.g., metal-core PCBs) for power-intensive equipment.
3. Manufacturing & Testing
Cleanroom assembly for implantable/wearable devices to prevent contamination.
Automated Optical Inspection (AOI) and electrical testing for defect-free production.
Long-term reliability testing (e.g., accelerated aging) for 10+ year lifespans.
4. Traceability & Documentation
Full component traceability (IPC-A-610 Class 3 standards).
Detailed manufacturing records for audits and recalls.
5. EMI/EMC & Signal Integrity
Shielding techniques to prevent interference in sensitive diagnostics.
Controlled impedance for high-frequency applications (e.g., ultrasound).
The medical PCB testing standards we C-alley follow:
We conduct continuous testing at every step of the manufacturing process to ensure the reliability of medical technology circuit boards.
Automatic Optical Inspection (A0): AOL uses a single 2D camera or two 3D cameras to capture images of PCBs. Compare the captured image with the schematic. If the circuit board does not match the schematic, send the circuit board for manual inspection.
Solderability testing: Ensure the reliability of solder joints.
Ion contamination testing: Perform ion contamination testing to detect ion residues generated during manufacturing and welding steps.
Peel off test: Determine the strength measurement required to peel off the laminated board from the circuit board.
Microsection analysis: Conduct inspections to determine the quality of the circuit board and identify internal faults.
Flying probe test: Move the test probe from the testing point to another testing point and check for open circuits, short circuits, and other issues.
Time Domain Reflectometer (TDR): TDR impedance measurement is crucial for ensuring signal integrity. It also checks for electrical interruptions in connectors or any other electrical paths.
How can we meet your high reliability requirements through full traceability?
According to IPC-6012 Class 2 and Class 3, we construct high-precision standard boards, advanced boards, and micro boards. Our advanced circuit boards comply with the UL 94V0 flammability standard. We provide the following documents and certificates.
certificate of conformity
Material specifications
First inspection report
Copy of reflux curve (included in the first article)
Photo requirements
IPC J-STD-001E
Record of all calibration tools used in the manufacturing process
AOI report or appearance inspection report
Flying needle or online testing report
Ionic Cleanliness Test Report
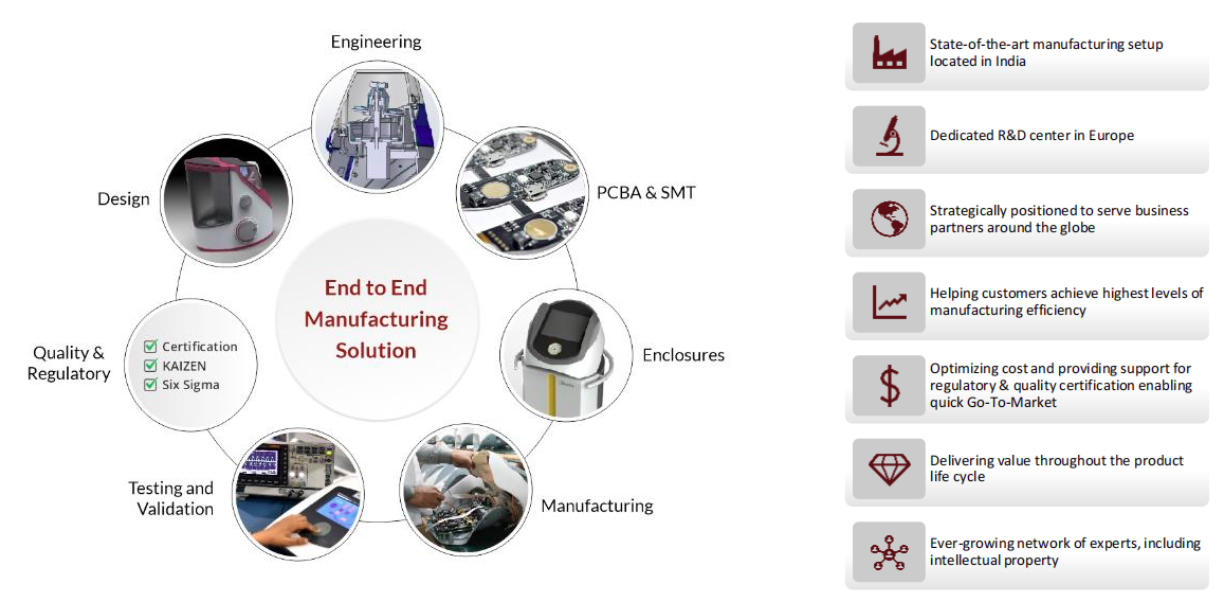
We follow the following standards to help us build high-quality and reliable medical PCBs for the healthcare and medical technology industries.
·IPC 6011: Define performance specifications for Class 1, Class 2, and Class 3 PCBs
IEC 60601-1: General requirements for the basic safety and essential performance of medical electrical equipment
IEC 60601-1-2: Define electromagnetic requirements (EMI and EMS) for medical PCBs
IS0 13485: Quality Management System Requirements for Providing Medical Equipment
We provide DFMIDFA inspection on each board
We focus on DEM and DFA analysis to prevent issues and provide an internal quality review system to ensure zero omissions. We also provide engineering support to verify whether your design operates as expected and complies with PC3 standards
Accurate, if you choose this standard
Extensive electrical testing of medical PCBs
We will not bargain over quality and adhere to the strict manufacturing standards required by the medical committee - this means that continuous testing must be conducted at every step of the manufacturing process, such as instrument loading, AO, BGAX radiation, flying pins, and other electrical tests.
One stop manufacturing and assembly
Poor communication between different manufacturing and assembly workshops for one-stop manufacturing and assembly may lead to delivery delays and circuit board errors. PCB manufacturers who do not handle assembly will not conduct DFA inspections to ensure that your circuit board can be assembled correctly. The supplier is responsible.
Get a Medical PCB Assembly Quote from C-alley
We need the following information to provide you with a quotation:
Gerber file
BOM LIST. If assembly is required.
Quantity
In Summary
Modern healthcare innovation drives the creation of advanced electronic medical equipment, addressing critical demands in patient care and clinical practice. These sophisticated systems serve essential functions ranging from diagnostic imaging to life-sustaining treatments, with implantable technologies like cardiac pacemakers demonstrating their vital role in global healthcare. Such medical electronics require precision-engineered circuit solutions to ensure reliability in clinical applications.
For healthcare technology developers seeking trusted PCB solutions, our specialized manufacturing services offer medical-grade quality assurance. Contact our technical team today for customized project consultation and competitive pricing.
Why choose C-alley?
C-alley delivers one-stop PCB manufacturing and assembly solutions tailored for the medical and health industry. We specialize in high-reliability PCBA services, ensuring stringent quality standards for life-critical applications.
What we can do for you:
Strong Engineering Service:
Software design
Hardware design
Electronic components selection,
Bom Optimization,
PCB LAYOUT
Prototype sample making
Software testing and adjustment
Qualified alternative recommend
Our mission is to provide cost-effective, precision-engineered medical PCB solutions without compromising performance or compliance.
If you seek a trusted partner for end-to-end medical PCB production—from prototyping to mass assembly—C-alley is your ideal choice. Start your new project with C-alley now.
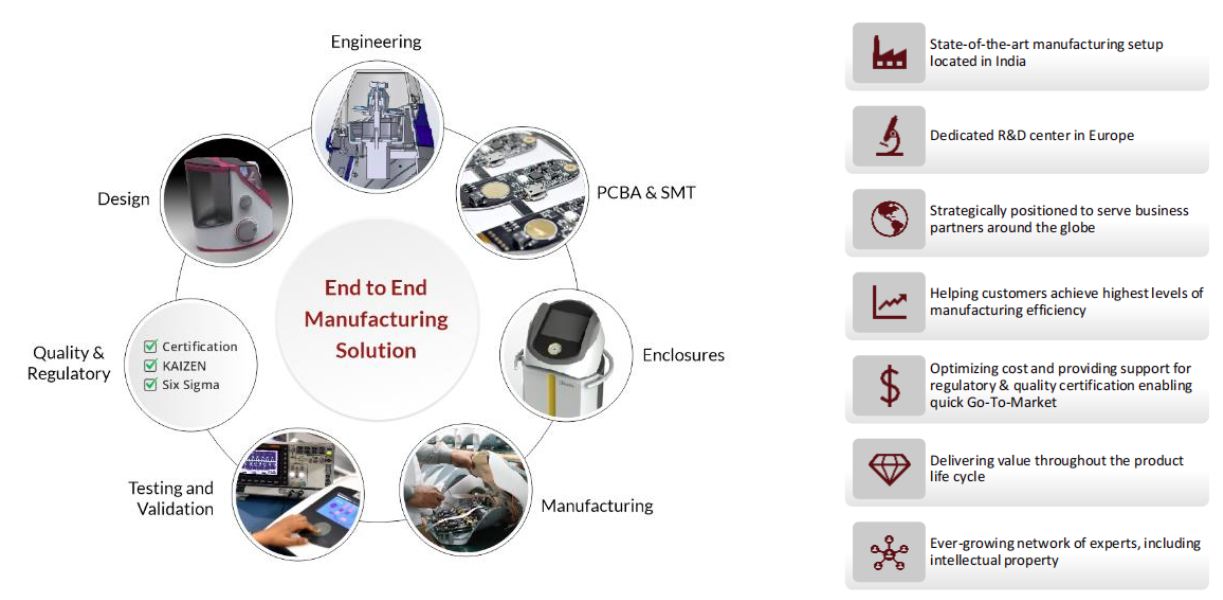
Please send Email to kspcba@c-alley.com or call us through +86 13828766801 Or submit your inquiry by online form. Please fill out below form and attach your manufacturing files( PCB Gerber files and BOM List) if need quotation. We will contact you shortly.
 +86 13828766801
+86 13828766801 kspcba@c-alley.com
kspcba@c-alley.com https://www.kingshengpcba.com/
https://www.kingshengpcba.com/ 2/F, Building 6, Tangtou 3rd Industrial Zone, Tangtou Community, Shiyan Town, Baoan District, Shenzhen, China, 518108
2/F, Building 6, Tangtou 3rd Industrial Zone, Tangtou Community, Shiyan Town, Baoan District, Shenzhen, China, 518108